
Revolutionizing
R&D Data Tsunami
with GenAI
A revolutionary bio-intelligence software platform empowering customers with automated clinical and regulatory intelligence so they can focus on what is most important for them – Strategy!

Revolutionizing
R&D Data Tsunami
with GenAI
A revolutionary bio-intelligence software platform empowering customers with automated clinical and regulatory intelligence so they can focus on what is most important for them – Strategy!

Revolutionizing
R&D Data Tsunami
with GenAI
A revolutionary bio-intelligence software platform empowering customers with automated clinical and regulatory intelligence so they can focus on what is most important for them – Strategy!
ria
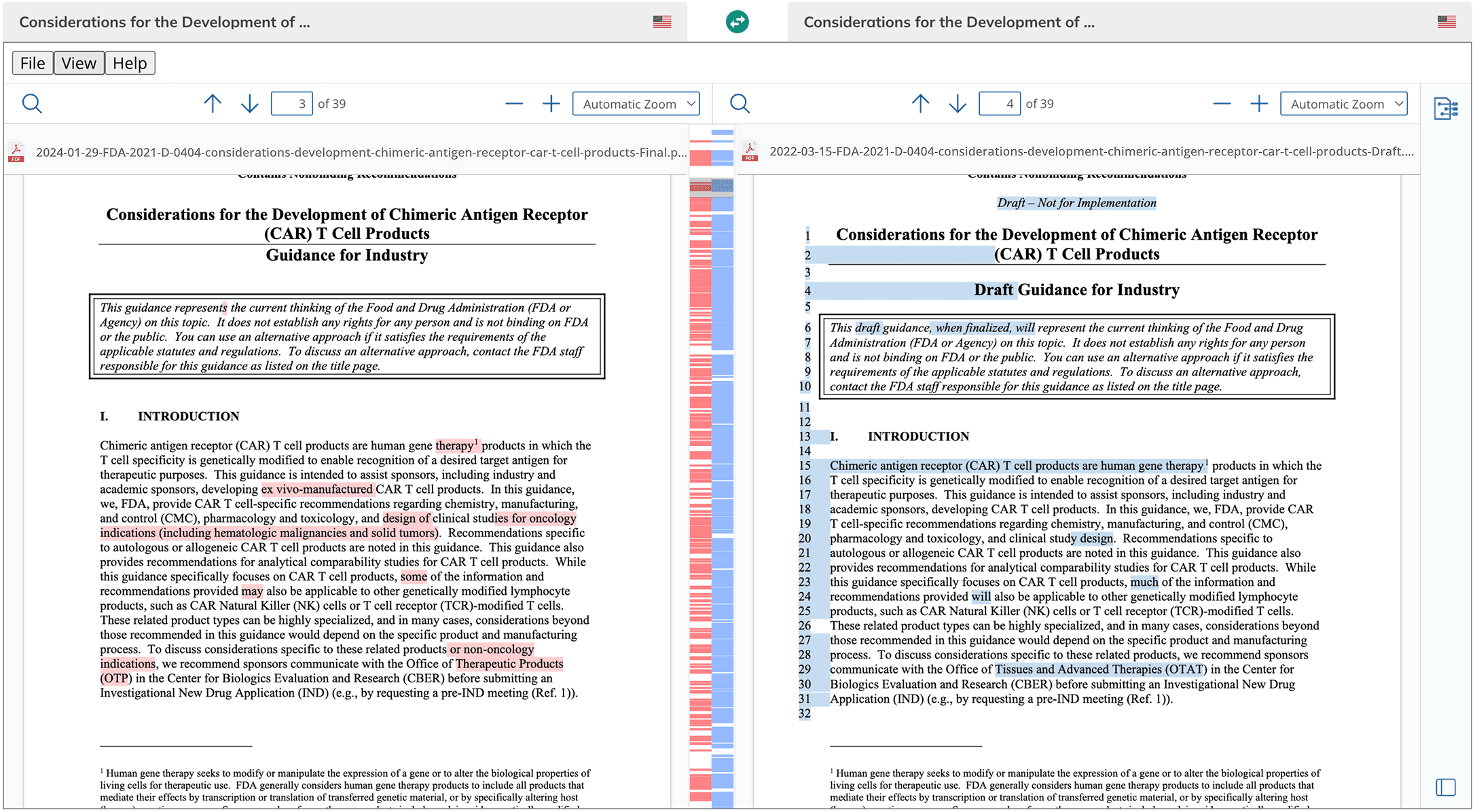
RIA (R&D Intelligence Assistant) provides access to global approval landscapes and submission requirements to labeling insights and application transitions. RIA delivers precise, research-backed answers—so you can move forward with confidence.


Democratizing Data
9
Agencies
150+
Data Sources
300K+
Drugs, Biologics & Devices
600K+
Clinical Trials
50MM+
R&D Pages
Value offered by RIA - Every insight, accelerated by intelligence!
Conduct Precedence Analysis with our
Subject Matter AI Expert
Conduct Precedence Analysis with our
Subject Matter AI Expert
RIA helps you
Find needle in a haystack
RIA Eliminates
Time consuming manual research and sifting through endless documents
create a summary of applications using "project orbis"
AI Response
Overview of Project Orbis Applications and Participating Countries
Project Orbis is an initiative led by the US FDA Oncology Center of Excellence to facilitate concurrent submission and review of oncology products among international regulatory partners. The program aims to expedite patient access to innovative cancer treatments across multiple countries by enabling collaborative, parallel review processes.
Below is a summary of applications utilizing Project Orbis and the countries participating in these collaborative reviews, based on the provided regulatory correspondence.
Applications Using Project Orbis
Trametinib Dimethyl Sulfoxide and Dabrafenib Mesylate (Novartis)
Indications: Pediatric glioma and other BRAF-mutant cancers.
Project Orbis Participation: Novartis was recommended to submit these applications via Project Orbis for both trametinib and dabrafenib, including pediatric formulations.
Countries Involved: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom (with the specific plan for this application including Brazil, Israel, Singapore, and Switzerland due to local filing
strategies)
1
2
Asparaginase Erwinia Chrysanthemi (Recombinant)-Rywn (Jazz Pharmaceuticals)
Indication: Acute lymphoblastic leukemia/lymphoma.
Project Orbis Participation: The FDA discussed the application with Project Orbis partners, and several countries expressed interest in reviewing under Project Orbis Type B or C submission plans.
Countries Involved: Australia, Canada, Singapore, Switzerland (with the sponsor to confirm timelines and
strategies)
3
Asciminib Hydrochloride (Novartis)
Indication: Chronic myeloid leukemia (CML), including T315I mutation.
Project Orbis Participation: FDA recommended Project Orbis participation, pending a global submission plan.
Countries Involved: Australia, Brazil, Canada, Singapore, Switzerland, United Kingdom
4
5
Quizartinib Dihydrochloride (Daiichi Sankyo)
Indication: Acute myeloid leukemia (FLT3-ITD positive).
Project Orbis Participation: FDA recommended consideration of Project Orbis, requiring a global submission plan.
Countries Involved: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom
6
Datopotamab Deruxtecan-DLNK
Indication: Non-small cell lung cancer (NSCLC) and other solid tumors.
Project Orbis Participation: FDA confirmed that multiple applications for the same product can qualify for Project Orbis, and recommended submission of a global submission plan.
Countries Involved: Not explicitly listed in the excerpt, but Project Orbis typically includes Australia, Brazil,
Canada, Israel, Singapore, Switzerland, United Kingdom
7
Datopotamab Deruxtecan-DLNK
Indication: Non-small cell lung cancer (NSCLC) and other solid tumors.
Project Orbis Participation: FDA confirmed that multiple applications for the same product can qualify for Project Orbis, and recommended submission of a global submission plan.
Countries Involved: Not explicitly listed in the excerpt, but Project Orbis typically includes Australia, Brazil,
Canada, Israel, Singapore, Switzerland, United Kingdom
8
Talquetamab-Tgvs
Indication: Multiple myeloma.
Project Orbis Participation: Sponsor planned to participate in Project Orbis for the initial BLA submission; FDA requested a global submission plan to alert Project Orbis Partners.
Countries Involved: Seven Project Orbis Partners (not individually listed in the excerpt, but consistent with
the standard group: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom)
9
Axatilimab-CSFR
Indication: Not specified in the excerpt, but related to oncology.
Project Orbis Participation: FDA recommended use of Project Orbis and submission of a global submission plan.
Countries Involved: Not explicitly listed, but aligns with standard Project Orbis partners
10
Executive Summary
Project Orbis is a collaborative regulatory initiative designed to accelerate access to innovative oncology therapies through simultaneous review by multiple international agencies. Applications for drugs such as trametinib, dabrafenib, asparaginase, asciminib, quizartinib, datopotamab deruxtecan, talquetamab, and axatilimab have utilized or planned to utilize Project Orbis. The core participating countries are Australia, Brazil, Canada, Israel, Singapore, Switzerland, and the United Kingdom. Participation requires a global submission plan and, often, additional documentation to support joint review. This approach streamlines regulatory processes and aims to bring life-saving therapies to patients more quickly across multiple regions
create a summary of applications using "project orbis"
AI Response
Overview of Project Orbis Applications and Participating Countries
Project Orbis is an initiative led by the US FDA Oncology Center of Excellence to facilitate concurrent submission and review of oncology products among international regulatory partners. The program aims to expedite patient access to innovative cancer treatments across multiple countries by enabling collaborative, parallel review processes.
Below is a summary of applications utilizing Project Orbis and the countries participating in these collaborative reviews, based on the provided regulatory correspondence.
Applications Using Project Orbis
Trametinib Dimethyl Sulfoxide and Dabrafenib Mesylate (Novartis)
Indications: Pediatric glioma and other BRAF-mutant cancers.
Project Orbis Participation: Novartis was recommended to submit these applications via Project Orbis for both trametinib and dabrafenib, including pediatric formulations.
Countries Involved: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom (with the specific plan for this application including Brazil, Israel, Singapore, and Switzerland due to local filing
strategies)
1
2
Asparaginase Erwinia Chrysanthemi (Recombinant)-Rywn (Jazz Pharmaceuticals)
Indication: Acute lymphoblastic leukemia/lymphoma.
Project Orbis Participation: The FDA discussed the application with Project Orbis partners, and several countries expressed interest in reviewing under Project Orbis Type B or C submission plans.
Countries Involved: Australia, Canada, Singapore, Switzerland (with the sponsor to confirm timelines and
strategies)
3
Asciminib Hydrochloride (Novartis)
Indication: Chronic myeloid leukemia (CML), including T315I mutation.
Project Orbis Participation: FDA recommended Project Orbis participation, pending a global submission plan.
Countries Involved: Australia, Brazil, Canada, Singapore, Switzerland, United Kingdom
4
5
Quizartinib Dihydrochloride (Daiichi Sankyo)
Indication: Acute myeloid leukemia (FLT3-ITD positive).
Project Orbis Participation: FDA recommended consideration of Project Orbis, requiring a global submission plan.
Countries Involved: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom
6
Datopotamab Deruxtecan-DLNK
Indication: Non-small cell lung cancer (NSCLC) and other solid tumors.
Project Orbis Participation: FDA confirmed that multiple applications for the same product can qualify for Project Orbis, and recommended submission of a global submission plan.
Countries Involved: Not explicitly listed in the excerpt, but Project Orbis typically includes Australia, Brazil,
Canada, Israel, Singapore, Switzerland, United Kingdom
7
Datopotamab Deruxtecan-DLNK
Indication: Non-small cell lung cancer (NSCLC) and other solid tumors.
Project Orbis Participation: FDA confirmed that multiple applications for the same product can qualify for Project Orbis, and recommended submission of a global submission plan.
Countries Involved: Not explicitly listed in the excerpt, but Project Orbis typically includes Australia, Brazil,
Canada, Israel, Singapore, Switzerland, United Kingdom
8
Talquetamab-Tgvs
Indication: Multiple myeloma.
Project Orbis Participation: Sponsor planned to participate in Project Orbis for the initial BLA submission; FDA requested a global submission plan to alert Project Orbis Partners.
Countries Involved: Seven Project Orbis Partners (not individually listed in the excerpt, but consistent with
the standard group: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom)
9
Axatilimab-CSFR
Indication: Not specified in the excerpt, but related to oncology.
Project Orbis Participation: FDA recommended use of Project Orbis and submission of a global submission plan.
Countries Involved: Not explicitly listed, but aligns with standard Project Orbis partners
10
Executive Summary
Project Orbis is a collaborative regulatory initiative designed to accelerate access to innovative oncology therapies through simultaneous review by multiple international agencies. Applications for drugs such as trametinib, dabrafenib, asparaginase, asciminib, quizartinib, datopotamab deruxtecan, talquetamab, and axatilimab have utilized or planned to utilize Project Orbis. The core participating countries are Australia, Brazil, Canada, Israel, Singapore, Switzerland, and the United Kingdom. Participation requires a global submission plan and, often, additional documentation to support joint review. This approach streamlines regulatory processes and aims to bring life-saving therapies to patients more quickly across multiple regions
create a summary of applications using "project orbis"
AI Response
Overview of Project Orbis Applications and Participating Countries
Project Orbis is an initiative led by the US FDA Oncology Center of Excellence to facilitate concurrent submission and review of oncology products among international regulatory partners. The program aims to expedite patient access to innovative cancer treatments across multiple countries by enabling collaborative, parallel review processes.
Below is a summary of applications utilizing Project Orbis and the countries participating in these collaborative reviews, based on the provided regulatory correspondence.
Applications Using Project Orbis
Trametinib Dimethyl Sulfoxide and Dabrafenib Mesylate (Novartis)
Indications: Pediatric glioma and other BRAF-mutant cancers.
Project Orbis Participation: Novartis was recommended to submit these applications via Project Orbis for both trametinib and dabrafenib, including pediatric formulations.
Countries Involved: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom (with the specific plan for this application including Brazil, Israel, Singapore, and Switzerland due to local filing
Asparaginase Erwinia Chrysanthemi (Recombinant)-Rywn (Jazz Pharmaceuticals)
Indication: Acute lymphoblastic leukemia/lymphoma.
Project Orbis Participation: The FDA discussed the application with Project Orbis partners, and several countries expressed interest in reviewing under Project Orbis Type B or C submission plans.
Countries Involved: Australia, Canada, Singapore, Switzerland (with the sponsor to confirm timelines and
strategies)
3
Asciminib Hydrochloride (Novartis)
Indication: Chronic myeloid leukemia (CML), including T315I mutation.
Project Orbis Participation: FDA recommended Project Orbis participation, pending a global submission plan.
Countries Involved: Australia, Brazil, Canada, Singapore, Switzerland, United Kingdom
4
5
Quizartinib Dihydrochloride (Daiichi Sankyo)
Indication: Acute myeloid leukemia (FLT3-ITD positive).
Project Orbis Participation: FDA recommended consideration of Project Orbis, requiring a global submission plan.
Countries Involved: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom
6
Datopotamab Deruxtecan-DLNK
Indication: Non-small cell lung cancer (NSCLC) and other solid tumors.
Project Orbis Participation: FDA confirmed that multiple applications for the same product can qualify for Project Orbis, and recommended submission of a global submission plan.
Countries Involved: Not explicitly listed in the excerpt, but Project Orbis typically includes Australia, Brazil,
Canada, Israel, Singapore, Switzerland, United Kingdom
7
Datopotamab Deruxtecan-DLNK
Indication: Non-small cell lung cancer (NSCLC) and other solid tumors.
Project Orbis Participation: FDA confirmed that multiple applications for the same product can qualify for Project Orbis, and recommended submission of a global submission plan.
Countries Involved: Not explicitly listed in the excerpt, but Project Orbis typically includes Australia, Brazil,
Canada, Israel, Singapore, Switzerland, United Kingdom
8
Talquetamab-Tgvs
Indication: Multiple myeloma.
Project Orbis Participation: Sponsor planned to participate in Project Orbis for the initial BLA submission; FDA requested a global submission plan to alert Project Orbis Partners.
Countries Involved: Seven Project Orbis Partners (not individually listed in the excerpt, but consistent with
the standard group: Australia, Brazil, Canada, Israel, Singapore, Switzerland, United Kingdom)
9
Axatilimab-CSFR
Indication: Not specified in the excerpt, but related to oncology.
Project Orbis Participation: FDA recommended use of Project Orbis and submission of a global submission plan.
Countries Involved: Not explicitly listed, but aligns with standard Project Orbis partners
10
Executive Summary
Project Orbis is a collaborative regulatory initiative designed to accelerate access to innovative oncology therapies through simultaneous review by multiple international agencies. Applications for drugs such as trametinib, dabrafenib, asparaginase, asciminib, quizartinib, datopotamab deruxtecan, talquetamab, and axatilimab have utilized or planned to utilize Project Orbis. The core participating countries are Australia, Brazil, Canada, Israel, Singapore, Switzerland, and the United Kingdom. Participation requires a global submission plan and, often, additional documentation to support joint review. This approach streamlines regulatory processes and aims to bring life-saving therapies to patients more quickly across multiple regions
Track Regulatory Changes and
Get AI Insights
Track Regulatory Changes and Get Insights
- Powered by AI
RIA helps you
Stay informed with real-time updates on evolving global regulations
RIA Eliminates
Manual monitoring and delayed awareness of regulatory changes
Guidance Summary
FDA Releases Draft Guidance to Mitigate Sepsis Transmission Risks in Human Cell and Tissue Products
The U.S. Food and Drug Administration (FDA) has issued a Draft Guidance for Industry titled Recommendations to Reduce the Risk of Transmission of Disease Agents Associated with Sepsis by Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps), effective May 2, 2025. This guidance aims to enhance the safety protocols surrounding donor eligibility determinations, specifically addressing the risks associated with sepsis transmission through HCT/Ps.
This draft guidance serves as an update to the previously established August 2007 HCT/P guidance on donor eligibility and incorporates findings from more recent public health investigations, particularly those related to Mycobacterium tuberculosis (Mtb) infections linked to HCT/Ps. The FDA recognizes the urgency of these updates, necessitating stakeholder comments to refine the guidance further.
Key Recommendations include:
Establishments must conduct comprehensive screenings of donors for risk factors, clinical evidence, and physical signs of sepsis.
Any ineligible donor identified due to medical history, clinical observations, or physical evidence associated with sepsis must be documented and excluded.
Medical records should be reviewed thoroughly, and communication with primary care physicians is encouraged when assessing donor eligibility.
While specific tests for sepsis detection are not available, establishments should perform testing for pathogenic agents that could lead to sepsis as part of the eligibility determination process.
The guidance underscores the classification of sepsis as a relevant communicable disease agent (RCDAD), maintaining its relevance in 21 CFR 1271.3(r)(2) and addressing increased scrutiny on donor safety to prevent further infections. By refining these protocols, the FDA aims to safeguard recipients and ensure higher standards of donor eligibility assessment, responding directly to evolving public health needs.
Under this draft, feedback from industry stakeholders is vital, and the FDA encourages comments to be submitted electronically or in writing by the date specified in the Federal Register's notice of availability. This effort reflects the agency’s commitment to enhancing public health safety while adapting to scientific advancements and emerging health threats related to tissue and cellular transplants.
Verify
Report
US-FDA - Guidelines
1h ago
36 New Guidelines Added
3 more notifications
US-FDA - Guidelines
1h ago
36 New Guidelines Added
3 more notifications
New Alert
1h ago
36 New FDA Guidelines Added
Recommendations to Reduce the Risk of Transmission of Disease Agents Associated with Sepsis by Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps): Draft Draft Guidance for Industry (1)
New Guidance document by CBER was added
Guideline document FertiPro N.V. HSA-containing ART media - Procedural steps and scientific in.. was added
New Guidance document by CBER was added
+34 more
Guidance Summary
FDA Releases Draft Guidance to Mitigate Sepsis Transmission Risks in Human Cell and Tissue Products
The U.S. Food and Drug Administration (FDA) has issued a Draft Guidance for Industry titled Recommendations to Reduce the Risk of Transmission of Disease Agents Associated with Sepsis by Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps), effective May 2, 2025. This guidance aims to enhance the safety protocols surrounding donor eligibility determinations, specifically addressing the risks associated with sepsis transmission through HCT/Ps.
This draft guidance serves as an update to the previously established August 2007 HCT/P guidance on donor eligibility and incorporates findings from more recent public health investigations, particularly those related to Mycobacterium tuberculosis (Mtb) infections linked to HCT/Ps. The FDA recognizes the urgency of these updates, necessitating stakeholder comments to refine the guidance further.
Key Recommendations include:
Establishments must conduct comprehensive screenings of donors for risk factors, clinical evidence, and physical signs of sepsis.
Any ineligible donor identified due to medical history, clinical observations, or physical evidence associated with sepsis must be documented and excluded.
Medical records should be reviewed thoroughly, and communication with primary care physicians is encouraged when assessing donor eligibility.
While specific tests for sepsis detection are not available, establishments should perform testing for pathogenic agents that could lead to sepsis as part of the eligibility determination process.
The guidance underscores the classification of sepsis as a relevant communicable disease agent (RCDAD), maintaining its relevance in 21 CFR 1271.3(r)(2) and addressing increased scrutiny on donor safety to prevent further infections. By refining these protocols, the FDA aims to safeguard recipients and ensure higher standards of donor eligibility assessment, responding directly to evolving public health needs.
Under this draft, feedback from industry stakeholders is vital, and the FDA encourages comments to be submitted electronically or in writing by the date specified in the Federal Register's notice of availability. This effort reflects the agency’s commitment to enhancing public health safety while adapting to scientific advancements and emerging health threats related to tissue and cellular transplants.
Verify
Report
US-FDA - Guidelines
1h ago
36 New Guidelines Added
3 more notifications
US-FDA - Guidelines
1h ago
36 New Guidelines Added
3 more notifications
New Alert
1h ago
36 New FDA Guidelines Added
Recommendations to Reduce the Risk of Transmission of Disease Agents Associated with Sepsis by Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps): Draft Draft Guidance for Industry (1)
New Guidance document by CBER was added
Guideline document FertiPro N.V. HSA-containing ART media - Procedural steps and scientific in.. was added
New Guidance document by CBER was added
+34 more
Guidance Summary
FDA Releases Draft Guidance to Mitigate Sepsis Transmission Risks in Human Cell and Tissue Products
The U.S. Food and Drug Administration (FDA) has issued a Draft Guidance for Industry titled Recommendations to Reduce the Risk of Transmission of Disease Agents Associated with Sepsis by Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps), effective May 2, 2025. This guidance aims to enhance the safety protocols surrounding donor eligibility determinations, specifically addressing the risks associated with sepsis transmission through HCT/Ps.
This draft guidance serves as an update to the previously established August 2007 HCT/P guidance on donor eligibility and incorporates findings from more recent public health investigations, particularly those related to Mycobacterium tuberculosis (Mtb) infections linked to HCT/Ps. The FDA recognizes the urgency of these updates, necessitating stakeholder comments to refine the guidance further.
Key Recommendations include:
Establishments must conduct comprehensive screenings of donors for risk factors, clinical evidence, and physical signs of sepsis.
Any ineligible donor identified due to medical history, clinical observations, or physical evidence associated with sepsis must be documented and excluded.
Medical records should be reviewed thoroughly, and communication with primary care physicians is encouraged when assessing donor eligibility.
While specific tests for sepsis detection are not available, establishments should perform testing for pathogenic agents that could lead to sepsis as part of the eligibility determination process.
The guidance underscores the classification of sepsis as a relevant communicable disease agent (RCDAD), maintaining its relevance in 21 CFR 1271.3(r)(2) and addressing increased scrutiny on donor safety to prevent further infections. By refining these protocols, the FDA aims to safeguard recipients and ensure higher standards of donor eligibility assessment, responding directly to evolving public health needs.
Under this draft, feedback from industry stakeholders is vital, and the FDA encourages comments to be submitted electronically or in writing by the date specified in the Federal Register's notice of availability. This effort reflects the agency’s commitment to enhancing public health safety while adapting to scientific advancements and emerging health threats related to tissue and cellular transplants.
Verify
Report
US-FDA - Guidelines
1h ago
36 New Guidelines Added
3 more notifications
US-FDA - Guidelines
1h ago
36 New Guidelines Added
3 more notifications
New Alert
1h ago
36 New FDA Guidelines Added
Recommendations to Reduce the Risk of Transmission of Disease Agents Associated with Sepsis by Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps): Draft Draft Guidance for Industry (1)
New Guidance document by CBER was added
Guideline document FertiPro N.V. HSA-containing ART media - Procedural steps and scientific in.. was added
New Guidance document by CBER was added
+34 more
Landscape and Feasibility Analysis
Landscape and Feasibility Analysis
RIA helps you
Quickly identify relevant applications and clinical trials for feasibility analysis
RIA Eliminates
Hours of manual research across disconnected sources and scattered endpoints
Clinical Trials
Skin care behaviors among melanoma survivors and their families
The impact of a prevention program on sun risks in primary school in tropical french region
Ranibizumab as adjuvant therapy for the treatment of choroidal melanoma (cohort 2)
Galectin inhibitor (gr-md-02) and ipilimumab in patients with metastatic melanoma
Ido inhibitor study for relapsed or refractory solid tumors
Ranibizumab as adjuvant therapy for the treatment of choroidal melanoma (cohort 2)
Phase 2
Phase 3
Phase 2
Phase 1
Phase 1
Phase 1
Applications
Tecelra
·
Proleukin
·
Cotellic
·
Melanoma
Clinical Trials
Skin care behaviors among melanoma survivors and their families
The impact of a prevention program on sun risks in primary school in tropical french region
Ranibizumab as adjuvant therapy for the treatment of choroidal melanoma (cohort 2)
Galectin inhibitor (gr-md-02) and ipilimumab in patients with metastatic melanoma
Ido inhibitor study for relapsed or refractory solid tumors
Ranibizumab as adjuvant therapy for the treatment of choroidal melanoma (cohort 2)
Phase 2
Phase 3
Phase 2
Phase 1
Phase 1
Phase 1
Applications
Tecelra
·
Proleukin
·
Cotellic
·
Melanoma
Clinical Trials
Skin care behaviors among melanoma survivors and their families
The impact of a prevention program on sun risks in primary school in tropical french region
Ranibizumab as adjuvant therapy for the treatment of choroidal melanoma (cohort 2)
Galectin inhibitor (gr-md-02) and ipilimumab in patients with metastatic melanoma
Ido inhibitor study for relapsed or refractory solid tumors
Ranibizumab as adjuvant therapy for the treatment of choroidal melanoma (cohort 2)
Phase 2
Phase 3
Phase 2
Phase 1
Phase 1
Phase 1
Applications
Tecelra
·
Proleukin
·
Cotellic
·
Melanoma
Labeling - Comparing, Monitoring and Analyzing Powered by AI
Labeling - Comparing, Monitoring and Analyzing Powered by AI
RIA helps you
Instantly compare labels & extract meaningful regulatory distinctions
RIA Eliminates
Time-consuming manual reviews and static label tracking processes
Label Comparison
LABEL
Tecelra
ORIG-1
LABEL
Mektovi
ORIG-1
Comparison Summary
Summary of Substantive Differences Between Tecelra and Mektovi Labels
Indications and Patient Selection
Tecelra is indicated for adults with unresectable or metastatic synovial sarcoma, requiring prior chemotherapy, specific HLA-A*02 alleles, and MAGE-A4 antigen expression (confirmed by companion diagnostic). Approval is under accelerated approval based on response rate.
Mektovi is indicated for unresectable or metastatic melanoma with BRAF V600E/K mutations, used only in combination with encorafenib. Requires BRAF mutation testing.
Contraindications
Tecelra: Contraindicated in patients with HLA-A*02:05P genotype (requires genetic screening).
Mektovi: No contraindications listed.
Dosage and Administration
Tecelra: Single IV infusion of genetically modified autologous T cells after lymphodepleting chemotherapy; requires cryostorage, thawing, and strict identity checks.Mektovi: Oral tablet (45 mg BID), chronic dosing, always with encorafenib; extensive dose modification guidance for toxicities.
Verify
Report
Label Comparison
LABEL
Tecelra
ORIG-1
LABEL
Mektovi
ORIG-1
Comparison Summary
Summary of Substantive Differences Between Tecelra and Mektovi Labels
Indications and Patient Selection
Tecelra is indicated for adults with unresectable or metastatic synovial sarcoma, requiring prior chemotherapy, specific HLA-A*02 alleles, and MAGE-A4 antigen expression (confirmed by companion diagnostic). Approval is under accelerated approval based on response rate.
Mektovi is indicated for unresectable or metastatic melanoma with BRAF V600E/K mutations, used only in combination with encorafenib. Requires BRAF mutation testing.
Contraindications
Tecelra: Contraindicated in patients with HLA-A*02:05P genotype (requires genetic screening).
Mektovi: No contraindications listed.
Dosage and Administration
Tecelra: Single IV infusion of genetically modified autologous T cells after lymphodepleting chemotherapy; requires cryostorage, thawing, and strict identity checks.Mektovi: Oral tablet (45 mg BID), chronic dosing, always with encorafenib; extensive dose modification guidance for toxicities.
Verify
Report
Label Comparison
LABEL
Tecelra
ORIG-1
LABEL
Mektovi
ORIG-1
Comparison Summary
Summary of Substantive Differences Between Tecelra and Mektovi Labels
Indications and Patient Selection
Tecelra is indicated for adults with unresectable or metastatic synovial sarcoma, requiring prior chemotherapy, specific HLA-A*02 alleles, and MAGE-A4 antigen expression (confirmed by companion diagnostic). Approval is under accelerated approval based on response rate.
Mektovi is indicated for unresectable or metastatic melanoma with BRAF V600E/K mutations, used only in combination with encorafenib. Requires BRAF mutation testing.
Contraindications
Tecelra: Contraindicated in patients with HLA-A*02:05P genotype (requires genetic screening).
Mektovi: No contraindications listed.
Dosage and Administration
Tecelra: Single IV infusion of genetically modified autologous T cells after lymphodepleting chemotherapy; requires cryostorage, thawing, and strict identity checks.Mektovi: Oral tablet (45 mg BID), chronic dosing, always with encorafenib; extensive dose modification guidance for toxicities.
Verify
Report
RIA Innovative Features
RIA streamlines regulatory intelligence with AI-powered search, intuitive analytics, and seamless document comparisons—helping you make faster, informed decisions
Natural Language search on complex documents
Find examples where US FDA approved products solely based on foreign clinical data
Find examples where US FDA approved products solely based on foreign clinical data
Search
Search
Searching from 107855 documents and 2,710,245 pages
Searching from 107855 documents and 2,710,245 pages
Tecelra
Tecelra
·
·
Proleukin
Proleukin
·
·
Proleukin
Proleukin
·
·
Cotellic
Cotellic
·
·
Intuitive Search Functionality and
Industry Best User Experience
Expedite your understanding with our AI Assistant - ChatRIA
N
You
Please summarize the efficacy study design for renal cell carcinoma
AI Response
The efficacy study design for renal cell carcinoma involved a multicenter, randomized, double-blind, international study called BO17705. The study compared the combination of interferon alfa and bevacizumab with interferon alfa and placebo in treatment-naïve metastatic renal cell carcinoma patients who had undergone a nephrectomy. A total of 649 patients were randomized in a 1:1 ratio to receive either bevacizumab (10 mg/kg every 2 weeks) or placebo with interferon alfa (9 MIU subcutaneously three times weekly for a maximum of 52 weeks). Patients were treated until disease progression or unacceptable toxicity. The main outcome measure was investigator-assessed progression-free survival (PFS), with overall survival (OS) as a secondary outcome measure. The study found that PFS was significantly longer in the bevacizumab group compared to the placebo group, but there was no significant improvement in OS.
Verify
Report
1/2
N
You
Please summarize the efficacy study design for renal cell carcinoma
AI Response
The efficacy study design for renal cell carcinoma involved a multicenter, randomized, double-blind, international study called BO17705. The study compared the combination of interferon alfa and bevacizumab with interferon alfa and placebo in treatment-naïve metastatic renal cell carcinoma patients who had undergone a nephrectomy. A total of 649 patients were randomized in a 1:1 ratio to receive either bevacizumab (10 mg/kg every 2 weeks) or placebo with interferon alfa (9 MIU subcutaneously three times weekly for a maximum of 52 weeks). Patients were treated until disease progression or unacceptable toxicity. The main outcome measure was investigator-assessed progression-free survival (PFS), with overall survival (OS) as a secondary outcome measure. The study found that PFS was significantly longer in the bevacizumab group compared to the placebo group, but there was no significant improvement in OS.
Verify
Report
1/2
Advanced analytics such as
AdComm, Guidance, PMR/C, Pediatrics, CDP, & Expedited Pathways due to unique data model
Public
Consultation
Orphan
Designation
Compliance
Letter
Drug Development
Tool (DDT)
HTA
EMA Meetings
Pediatrics
PMR/PMC
Label
Comparison
Clinical Development
Plan (CDP)
Regulatory
360
Guideline
Advisory
Committee
Expedite
Pathway
Orphan
Designation
Public
Consultation
Orphan
Designation
Compliance
Letter
Drug Development
Tool (DDT)
HTA
EMA
Meetings
Pediatrics
PMR/PMC
Label
Comparison
Clinical
Development
Plan (CDP)
Regulatory
360
Guideline
Advisory
Committee
Expedite
Pathway
Orphan
Designation
Automate workflows to enhance efficiency, save time, and reduce errors.
Regulatory 360
Regulatory 360
Pediatrics
Pediatrics
Guideline
Guideline
EMA Meetings
EMA Meetings
Automation
investigator-assessed progression-free survival (PFS), with overall survival (OS) as a secondary
outcome measure. The study found that PFS was significantly longer in the bevacizumab group compared to the placebo group, but there was no significant improvement in OS.
investigator-assessed progression-free survival (PFS), with overall survival (OS) as a secondary
outcome measure. The study found that PFS was significantly longer in the bevacizumab group compared to the placebo group, but there was no significant improvement in OS.
Verify
Verify
Keep hands on the wheel
Verify & Use AI generated data
with assurance!
Product Label, Pediatric, & Guidance Comparisons


Hear it from Innovators
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
" The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
Senior Director Global Regulatory Intel


"With more than thirty years in regulatory affairs, I have not seen a solution transform intelligence gathering as Vivpro’s AI platform. Our long-standing collaboration is strengthened by the excellent white glove service the Vivpro team delivers; they anticipate our needs, respond quickly to every question, and listen to our feedback resulting in enhanced features to the platform. The insights we provide using Vivpro are trusted, and the time we save is reinvested in faster, better strategy decisions."
"RIA is a lot smarter than Siri."
Distinguished Scientist, Pumas AI


"I love how RIA helps me find information quickly. While working on an interaction model from an FDA summary, I simply asked RIA, 'What is the model of drug X and drug Y?' Within seconds, the exact model table I needed appeared. It's faster than searching through stored documents. RIA is a lot smarter than Siri."
Ready to Innovate?
Let’s start your journey toward innovation. Get in touch with us today to see how Vivpro can empower your team and drive your success.